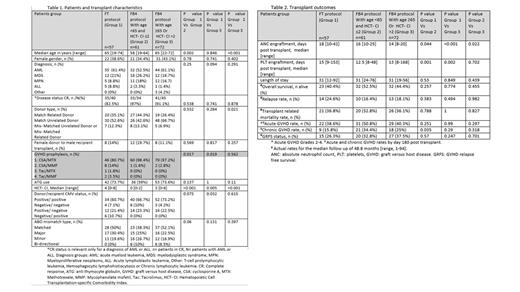
Background: Allogeneic hematopoietic stem cell transplantation (HSCT) is a potentially curative treatment for hematological malignancies. Myeloablative conditioning (MAC) results in a better disease-free survival (DFS) compared to reduced-intensity conditioning (RIC). However, the use of MAC in older adults or in those with comorbidities is limited due to a high rate of non-relapse mortality (NRM). Treosulfan-based conditioning regimens were found to result in superior DFS compared to RIC, without increased NRM. However, patients over the age of 65 were less represented in trials assessing the safety and efficacy of treosulfan-based conditioning relative to MAC. In recent years, fludarabine-treosulfan (FT) conditioning was used at Rambam for patients ≥65 years old, or for those with an HCT - comorbidity index (HCT-CI) score >2. This study aimed to evaluate the efficacy of the FT conditioning protocol and fludarabine-busulfan for 4 days (FB4) in older adults or in those with comorbidities. Methods: This single-center retrospective study included the following 3 groups: 1) patients who received the FT protocol; 2) patients aged <65 years with HCT-CI ≤2, who received the FB4 protocol; 3) patients aged ≥65 and/or with HCT-CI >2, who received the FB4 protocol. The results of patients included in group 2 were used as a reference. Data were retrieved from the electronic medical records. Baseline characteristics, transplant outcomes and complications were compared. Categorical variables and non-parametric variables were evaluated with the Fisher's exact test and Mann‐Whitney U test, respectively. Results: One hundred and ninety patients were analyzed. All underwent HSCT between January 2015 and December 2021 (table 1). The FT group, younger and older FB4 groups included 57, 61 and 72 patients, respectively. Patient median age was 65 years in both the FT and older FB4 groups, compared to 58 in the younger FB4 group (p<0.05). Patients in the FT group had significantly more comorbidities compared to younger FB4 (p<0.001) and older FB4 groups (p=0.005), with a median HCT-CI of 4, 0 and 3, respectively. During a median follow-up of 48.8 months, there were no significant differences between the groups in terms of the incidence of acute graft-versus-host disease (GVHD), disease relapse, NRM or overall survival (table 2). However, the chronic GVHD rate was 34.4% in the younger FB4 group and only 15.8% in the FT group (p=0.035). This rate was 25% in the older FB4 group (p=NS). Mucositis rate was significantly lower in the FT group, with 31.6% of patients being mucositis-free, compared to 6.6% and 13.9% in the younger and older FB4 groups, respectively. However, the rate of bacteremia events was significantly increased in the FT group (49.1%) relative to the younger FB4 (13.1%) and the older FB4 (23.6%) groups. Conclusions: In older patients or in those with comorbidities, FT appears to be as efficient as FB4 conditioning. Furthermore, these outcomes are comparable to those observed in younger patients conditioned with FB4. Hence, both of the evaluated regimens could be considered in these patient populations. Prospective randomized studies are warranted to further evaluate these findings.
Disclosures
Zuckerman:Orgenesis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BioSight Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Cellect Biotechnology: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal